Canine Urinary Bladder Cancer Research
Currently, more than 400,000 people in the United States have urinary bladder cancer. This disease detracts from quality of life and takes the lives of more than 16,000 people each year. Most deaths are due to the more aggressive form of bladder cancer, high grade, invasive transitional cell carcinoma (TCC). Fortunately, many people with bladder cancer have a less aggressive form of the disease (lower grade, superficial bladder cancer) that is usually not life threatening. Urinary bladder cancer also affects pet dogs. Unfortunately, most dogs with bladder cancer have intermediate to high grade invasive TCC. Urinary obstruction and spread of the cancer has taken the lives of most dogs with TCC.
As summarized below; however, we are making progress against this disease. In the Werling Comparative Oncology Research Center (WCORC), a unique combination of epidemiological work, clinical studies in dogs, and complementary laboratory research is being used to tackle urinary bladder cancer. Studies have allowed us to learn some of the causes of TCC in dogs, and this can lead to strategies to prevent the disease. More effective ways to treat TCC in dogs have been identified. These new treatment approaches have extended survival and improved the quality of life for dogs with TCC, and these studies in pet dogs have resulted in clinical trials in humans with bladder cancer.
Dr. Debbie Knapp on the Werling Comparative Oncology Research Center
Canine Bladder Cancer

Cancer of the urinary tract in dogs can affect the kidneys, ureters, urinary bladder, prostate, or urethra (see Figure 1). Within the urinary system, the bladder is the location most frequently affected with cancer. Compared to cancer in other locations in the body, bladder cancer is unusual, comprising approximately 2% of all cancers in the dog. With more than 70 million pet dogs in the United States, however, even unusual cancers like bladder cancer, are problems for thousands of dogs and their families.
Frequently Asked Questions by Pet Owners
WHAT IS BLADDER CANCER?
The most common cancer of the urinary bladder in dogs is invasive transitional cell carcinoma (TCC) of intermediate to high grade. This cancer is also called invasive urothelial carcinoma (InvUC), or in humans is usually called “muscle invasive bladder cancer” (MIBC). In this article, we will use the term more commonly recognized by pet owners, TCC. TCC is a malignant tumor that develops from the transitional epithelial cells that line the bladder. In dogs, this tumor invades into the deeper layers of the bladder wall including the muscle layers. As the cancer enlarges in the bladder, it can cause obstruction to the flow of urine from the kidneys to the bladder or from the bladder to the outside of the body. Canine TCC also has the ability to spread to lymph nodes and to other organs in the body (lung, liver, others). TCC most frequently is found in the bladder, but can also develop in the kidneys, ureters, prostate, and urethra. If we pause for a minute to consider human bladder cancer, the cancer in humans generally falls into two general categories: (1) lower grade, superficial tumors, and (2) higher grade, invasive tumors. It is fortunate that the majority of people with bladder cancer have the lower grade, superficial form of the disease, which typically does not spread beyond the bladder. Dogs, on the other hand, most often develop the higher grade, invasive form of bladder cancer that can grow more quickly and can spread throughout the body.
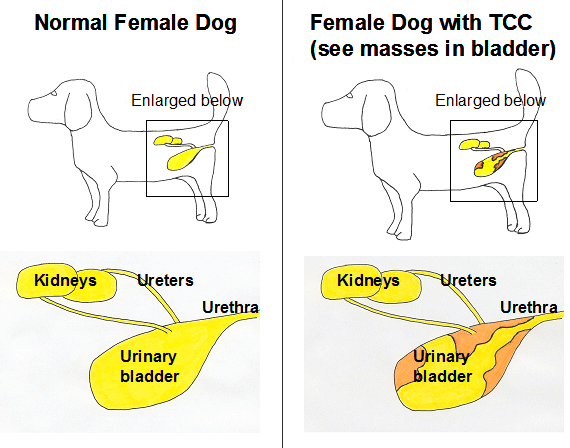
Figure 1
WHAT CAUSES TCC IN DOGS?
The exact cause of TCC in an individual dog is usually not known. In general, canine TCC results from a combination of several factors including genetic predisposition and environmental factors. A genetic predisposition is strongly suspected because TCC is more common in specific breeds of dogs. Scottish Terriers have an 18-20 fold higher risk of TCC than other dogs. Shetland Sheepdogs, Beagles, West Highland White Terriers, and Wire Hair Fox Terriers are 3 to 5 times more likely to develop TCC than other dogs. Other breeds of dogs considered at higher risk for TCC in some studies include Eskimo dogs, Keeshonds, and Samoyeds. Dogs in related breeds may also have a higher risk of TCC, but this has not been studied yet.
Environmental factors identified as risk factors in early studies have included pesticides and insecticides such as "old generation" flea dips. The greatest cause of TCC in humans is smoking. There is concern that second-hand smoke could contribute to TCC in dogs, as suggested in recent work.
An association has been found between exposure to lawn herbicides and pesticides and the risk of TCC in Scottish Terriers. Investigators at the Purdue University School of Veterinary Medicine published a case control study in Scottish Terriers to determine risk factors for the development of TCC. As discussed above, Scottish Terriers have an 18-20 times higher risk for developing TCC than dogs of other breeds. The study was performed to determine if exposure to certain types of environmental chemicals would further increase the risk of TCC in this breed of dog. Environmental exposure histories were compared between 83 Scottish Terriers with TCC (cases) and 83 Scottish Terriers of approximately the same age without bladder cancer (controls). A significantly increased risk of TCC was found for dogs exposed to lawns or gardens treated with herbicides and insecticides or herbicides alone. In fact, dogs exposed to treated lawns were seven times more likely to develop TCC. These findings indicate that Scottish Terriers, as well as other dogs of high-risk breeds for TCC, should be restricted from lawns treated with herbicides and pesticides. The risk of lawn chemicals to dogs in other breeds has not yet been determined.
WHAT CLINICAL SIGNS OR SYMPTOMS DO DOGS WITH TCC HAVE?
Blood in the urine, straining to urinate, and making repeated frequent attempts to urinate are the most common signs of TCC in dogs. Having urinary accidents in the house is also common. Pet owners must realize, however, that a urinary tract infection will cause these same symptoms, so the symptoms alone do not necessarily mean the dog has TCC. Less commonly, dogs with TCC can have lameness due to spread of the tumor into the bones or spread into the lungs and a paraneoplastic syndrome called hypertrophic osteopathy.
HOW IS TCC DIAGNOSED?
A definitive diagnosis of TCC requires a tissue biopsy. Several other types of growths in the bladder, bladder infection, bladder stones, or bladder inflammation can cause similar symptoms as those in dogs with TCC. Some of these other conditions can also cause "masses" to be seen on radiographs or ultrasound, and these other conditions can cause abnormal cells in the urine, which can be mistaken for TCC. Urine tests have been developed that detect a certain proprietary antigen (a specific protein) in the urine, or that detect a specific mutation in a gene called the BRAF gene. In a recent study, however, both of these types of tests had frequent false positive and false negative results (Read more at New study published on bladder cancer screening and early treatment in dogs). In other words the BRAF mutation was present in dogs that did not have cancer and that did not develop cancer. Therefore, to be certain if a dog has TCC or not, it is still necessary to obtain a tissue biopsy. This is important because the treatment and prognosis depend entirely on exactly what is wrong with the bladder. A tissue biopsy can be obtained by surgery, cystoscopy (insertion of a fiberoptic scope into the bladder and biopsy through the scope), or in some cases with a urinary catheter.
WHAT EVALUATION IS NEEDED FOR A DOG WITH TCC?
Once a diagnosis of TCC is made, it is important to determine the extent of the tumor, i.e. to perform "tumor staging". Tumor staging is performed to determine the best way to treat the cancer, to provide some information regarding prognosis, and to establish a baseline set of tumor measurements in order to determine if subsequent treatment is being successful. Tumor staging for TCC includes radiographs (“x-rays”) of the thorax to look for lung metastasis, radiographs and ultrasound (or CT scan) of the abdomen to look for metastasis in the abdomen and to assess any changes in the kidneys that result from obstructed urine flow, and imaging of the bladder to determine the exact location and size of the tumor within the bladder (see Figure above). This information is needed to best plan how to treat the cancer. Also, these tests are repeated during treatment to know if the treatment is being effective. When ultrasound is used to measure the tumor size and how well the treatment is working over time, it is critical to use a standardized imaging protocol. At Purdue this typically includes ultrasonography with the same ultrasound operator and machine, the same dog position (lying on the right side), the same level of bladder fullness, and acquisition of images from the same plane of ultrasound probe angle.
HOW IS TCC TREATED?
Surgery
For dogs with TCC that has not spread beyond the bladder, surgical excision could be considered. In order to surgically excise the tumor, however, it needs to be located away from the neck (also called the trigone) of the bladder and the urethra. Several vital structures in the neck of the bladder (junction with ureters and urethra, urethral sphincter) usually prevent surgical excision of tumors in this location. This is especially true because malignant tumors, like TCC, need to be removed with a "margin" of normal tissue around the tumor. This "margin" often contains microscopic tumor cells that, if left behind, would result in cancer regrowth. In addition, most canine TCCs invade down into the bladder wall and therefore, surgical excision requires removal of a complete full thickness section of bladder wall. [Note: in humans with superficial, low grade cancer, this is not typically the case.] Because most canine TCCs are invasive into the bladder wall and located in the neck of the bladder, surgical removal is usually not possible.
Radiation therapy
If surgery is not possible, what other treatment options are available? Radiation therapy has been used to successfully control TCC growth in the bladder in dogs. Unfortunately, radiation given in traditional doses when applied to the bladder can lead to harmful complications including a scarred, shrunken bladder, and irritation to surrounding organs. One of the challenges in applying radiation to the bladder is that the bladder can move or flop within the abdomen and take on a different shape depending on how much urine is in the bladder and if structures next to the bladder such as the bowel are pushing in on the bladder. With better imaging, radiation therapy of the bladder has become much safer than in years past. The optimal treatment protocols (doses, frequency) and the level of benefit offered by radiation therapy require further study.
Drugs
The vast majority of dogs with TCC are treated with medical therapy, i.e. with drugs. There are at least 10-12 drugs that can be helpful in treating TCC. No one drug stands out as the very best over the others. The pet owner and veterinarian should discuss the treatment options and select the starting treatment that the dog owner is the most comfortable with. Some information on the more commonly used treatments is provided here.
Piroxicam and other NSAIDs
A conservative oral treatment is a drug called piroxicam, or a piroxicam-like drug by itself. Piroxicam is a type of drug called a nonsteroidal antiinflammatory drug or “NSAID”. NSAIDs block the cyclooxygenase (cox) enzyme, and are also referred to as “cox inhibitors”. Cox inhibitors include piroxicam, aspirin, ibuprofen, Previcox, Deramaxx, Rimadyl, and others. [Please do not give dogs ibuprofen as dogs do not tolerate this drug as well as humans do.] There is an interesting history behind the use of cox inhibitors for the treatment of TCC in dogs. Veterinarians in the WCORC and a veterinarian colleague (Dr. T Needham, Wilmington N.C.) became interested in piroxicam several years ago when it was being used for pain relief in dogs with cancer, and unexpected remissions were noted. Two of the first dogs treated had advanced cancer (one with metastatic carcinoma, one with undifferentiated sarcoma), and these dogs had remission of their cancer when receiving piroxicam, but no other treatment. This has led to numerous studies of piroxicam in animals with cancer at Purdue. In 76 dogs with TCC treated with piroxicam, the tumor went into complete remission in 2 dogs, decreased in size by > 50% in 14 dogs, remained "stable" in size (<50% change) in 45 dogs, and increased in size by > 50% in 15 dogs. Although remission is certainly the preferred treatment outcome, “stable disease” is also considered a beneficial response when the dog is feeling well and enjoying life. In that scenario, the cancer is “managed” as a chronic disease, and the dog lives with it. The median (“average”) survival of the dogs treated with piroxicam was 244 days. It should be noted that this survival was from the time piroxicam was started until the dog died. Some dogs received other medications before piroxicam or after the cancer became resistant to piroxicam, and these drugs could have affected survival. Another important clarification to make is that while the median survival is reported here, the survival varies tremendously from dog to dog. In fact, the survival ranged from 6 days to 1256 days for dogs treated with piroxicam! For dogs receiving piroxicam, it is important for pet owners to observe the dog and make note of loss of appetite, unexplained vomiting, and dark “tarry” looking stools (melena). These could indicate stomach and intestinal irritation resulting from the piroxicam. In this situation, piroxicam should be stopped until the dog returns to normal. The pet owner should talk to their veterinarian about whether to re-start piroxicam or to switch to a different medication.
Vinblastine and NSAIDs
Another treatment option is to combine piroxicam (or a piroxicam-like drug) with an intravenous medicine called vinblastine. Vinblastine is a chemotherapy drug that is typically given intravenously at 2-week intervals in dogs with TCC. Dogs receiving vinblastine and piroxicam together as their first treatment had the following tumor responses: 58% partial remission, 33% stable disease, and 8% progressive disease with a median survival of 299 days (range 21-637 days). Vinblastine and piroxicam can also be used after other treatments have failed, although the remission rate is not as high in that setting. Vinblastine is generally well-tolerated with severe side effects being uncommon. This protocol requires more frequent veterinary visits than piroxicam alone. Most dogs see a veterinarian weekly while receiving vinblastine and piroxicam, including visits for blood counts on non-treatment weeks.
Other chemotherapy drugs and NSAIDs
Another treatment protocol that was considered the “chemotherapy protocol of choice” prior to the vinblastine studies involves a combination of piroxicam and an intravenous chemotherapy drug called mitoxantrone. In a study performed by the Veterinary Cooperative Oncology Group, this combination treatment resulted in a remission rate of approximately 35%. In addition to dogs that had remission, 46% of the dogs also had “stable disease” where the cancer did not grow substantially for a period of time. “Average” survival times with mitoxantrone/piroxicam have been in the 250-300 day range. As with other drugs, some dogs live much longer than this, while others do not live this long. At Purdue, mitoxantrone is one of the drugs given as “second line” treatment if vinblastine does not work, or stops working over time. Another drug used in this “second line” or “third line” treatment is an intravenous chemotherapy called carboplatin.
Metronomic chemotherapy
Another option for treating TCC is “metronomic” chemotherapy. The term metronomic chemotherapy is used to describe the frequent (typically daily), low dose, oral administration of chemotherapy. At these low doses, the drug is thought to work by modulating the immune response, disrupting tumor blood flow, or other mechanisms. The expected outcome of metronomic chemotherapy is that the cancer will stop growing for a period of time (ideally for many months or more). The cancer is not expected to shrink, but to stabilize in growth. Briefly, in a study at Purdue University, a series of 31 dogs with TCC were treated with low dose oral chlorambucil (also called leukeran). In the study, one dog had remission, and 20 dogs had stable disease, for a cancer control rate of 70%. This was encouraging since the cancer in all but 2 of the dogs had already developed resistance to other therapies. The median survival was 221 days (range 7-7474 days). Side effects of the treatment are uncommon in the initial stages. After some months, however, chronic suppression of the bone marrow can occur resulting in low platelet or white blood cell counts. This observation of chronic low blood counts has dampened the enthusiasm for metronomic chemotherapy in dogs. In addition, the veterinarian and dog owner should discuss whether “cancer control”, rather than cancer shrink is an appropriate goal for an individual dog.
Intravesical therapy
Pet owners that know people with bladder cancer often ask why intravesical therapy is not done more often in dogs with TCC. Intravesical therapy, which refers to placing anticancer drugs directly into the bladder though a urinary catheter, is a mainstay in the treatment of humans with superficial TCC. The drug is expected to stay in the bladder where high concentrations can come in direct contact with the cancer. Initially, it was not known if intravesical therapy would be of benefit in dogs because TCC in dogs would be deeper in the bladder wall, tumor masses would often be larger than those treated in humans, and the drug might not reach enough of the tumor. A clinical trial of intravesical therapy (specifically intravesical mitomycin C) in 12 dogs with TCC at Purdue University revealed two important findings. The first finding was that the antitumor effects were encouraging, consisting of partial remission in 5 dogs and stable disease in 7 dogs. Unfortunately, the second important finding was that in 2 dogs, the drug appeared to pass from the bladder into the blood stream and then throughout the body. These dogs had severe side effects similar to what would occur with high dose intravenous chemotherapy. Although both dogs recovered, if a larger amount of drug were to be absorbed into the blood stream, it could be lethal. For this reason, intravesical mitomycin C therapy is not typically given to dogs. Our group has also studied other intravesical drugs, although more study is required to determine if these will have a role in standard therapy.
Monitoring plan
Regardless of the treatment pursued, the typical plan followed in most dogs with TCC at Purdue University is to measure the extent of the tumor before treatment, and then to remeasure the tumor after 4-8 weeks of treatment (depending on the drug used). Measuring the tumor is important because the dog’s clinical signs, i.e. “symptoms” do not always follow changes in tumor size. If the dog develops a urinary tract infection, their symptoms will be much worse, yet the tumor might not be growing. Monitoring changes in gene mutations such as the BRAF gene in the urine, also fails to predict changes in tumor size. In addition to following changes in tumor size, various tests are also used to detect any side effects. If the tumor is shrinking or remaining stable in size after 4-8 weeks, and the dog is feeling well on that therapy, then the same treatment is continued. If the cancer is not responding, i.e. if it is growing, or if the dog does not feel well on that particular treatment, then a different treatment is initiated. After each 4-8 weeks of treatment the tumor is remeasured to confirm the treatment is still being beneficial, and to alter therapy as indicated.
ARE SIDE EFFECTS COMMON WITH THE CANCER DRUGS USED IN DOGS?
Many pet owners have observed humans undergoing chemotherapy and are concerned that some of the serious side effects of chemotherapy in humans will also occur in their pet dogs. Fortunately, most dogs treated with chemotherapy, experience much less toxicity than humans receiving chemotherapy. This may include one day every 2-3 weeks in which the dog may be a less active, eat less, or have a mild upset stomach. For a minority of dogs, e.g. <5%, the side effects could be much worse. Although we cannot predict ahead of time which dogs will have severe side effects, if those side effects occur, we do not recommend continuing that treatment for that dogs. Cox inhibitors like piroxicam have few side effects. In some dogs (<20%), however, piroxicam will irritate the stomach or intestine. Therefore, if a dog on piroxicam has loss of appetite, vomiting, or dark tarry-looking stools, it is safest to stop the piroxicam and consult the veterinarian before starting the medication again. The new cox inhibitors, selective cox-2 inhibitors, such as deracoxib, meloxicam, and firocoxib, are not expected to cause stomach irritation as frequently as piroxicam does. Treatment protocols are selected with the goal of maintaining or improving quality of life, at the same time the cancer is attacked. However, nothing in medicine is risk free, and the pet owner should discuss the possible benefits and risk of specific medications that their dog may receive with the attending veterinarian.
WHAT ARE THE ADVANTAGES OF CLINICAL TRIALS?
In order to improve the outlook for dogs and humans with invasive bladder cancer, the WCORC is conducting clinical trials in dogs with TCC. Dogs that take advantage of the clinical trials as well as standard care over the course of their cancer, can have longer survival, typically well beyond a year for many dogs. Clinical trials in dogs are similar to clinical trials in humans. The dogs live at home with their families, and come into the Purdue University Veterinary Hospital periodically for evaluation and treatment. Quality of life for the dogs is the highest priority, so treatments evaluated in clinical trials are selected with the goal of having antitumor effects with low risk of serious side effects. The advantages for a dog participating in a clinical trial are that the dog is receiving treatment that is expected to be as effective or more effective than standard therapies, the dog is helping veterinarians learn important information that is expected to help other dogs and even humans with bladder cancer, and the dog is receiving some “hope” if standard therapy is not an option or has failed. In some instances, participating in a treatment trial is less expensive than other treatments.
WHAT IS THE PROGNOSIS FOR DOGS WITH TCC?
In early studies, survival in dogs with TCC was listed as "0 days". At that time, it was thought there was "no hope", and many dogs were euthanized at the time of diagnosis. It is not known how long dogs with TCC that are not treated will live. Survival is affected by the growth rate of the tumor, the exact location of the tumor within the bladder, and whether the tumor has spread to other organs or not. The median (“average”) survival in 55 dogs treated with surgery alone (before drugs that could help were identified) was 109 days. The median survival in dogs treated with early chemotherapy alone (cisplatin or carboplatin) at Purdue University was 130 days. Median survival with piroxicam treatment in 76 dogs with TCC was 244 days. The median survival of dogs receiving vinblastine and piroxicam was 299 days. As mentioned above, approximately 35% of dogs receiving mitoxantrone and piroxicam have remission, and the average survival is around 250-300 days. The survival times in all of these studies, however, varied tremendously from dog to dog. Some dogs with advanced cancer died after only a few days, while others lived more than two years. As mentioned above, dogs who live the longest are often those that receive more than one treatment protocol (one after the other switching therapies when the cancer begins to grow) during the course of the cancer. Other dogs that survive long periods of time appear to inherently have more responsive tumors. Factors that have been identified in our studies that negatively affect survival time include more extensive tumor within the bladder, spread of tumor beyond the bladder, and involvement of the tumor in the prostate gland. Regarding metastasis of TCC in dogs, approximately 20% of dogs with TCC have detectable metastasis at diagnosis, and 50-60% have metastasis at death.
Although progress has been made, and TCC is considered a very “treatable” disease, there is still much to be learned. We are not satisfied with the “efficacy” of current therapy, especially long term. Therefore, we are continuing to study TCC to determine better ways to prevent, manage, and treat this cancer. Learn more about our ongoing clinical trials.
WHAT SYMPTOMATIC CARE CAN BE GIVEN TO DOGS WITH TCC?
Treating secondary infections
Dogs with TCC are more likely to develop a urinary tract infection (cystitis) than dogs without cancer. A secondary bacterial infection can result in a sudden worsening in symptoms (blood in urine, straining to urinate, urinary accidents) in dogs with TCC, and these dogs can improve with treatment with antibiotics. A frustrating aspect of urinary tract infections that is being encountered more often in recent years is the development of bacterial infections that are resistant to commonly used antibiotics. This is one reason to not “over use” antibiotics, as this can lead to more bacterial resistance. In addition, antibiotics will change the “natural bacteria” within the body and cause changes in the body’s “microbiome”. The microbiome which consist of microorganisms that normally live in the intestinal tract, in the urinary tract, and other areas of the body can have a strong influence on the immune response to cancer. Changing the microbiome can have detrimental effects, and blunt the immune attack on the cancer. For all of these reasons, antibiotics should be reserved for dogs with evidence of infection found on urinalysis. And, the antibiotics should ideally be selected based on culture and sensitivity testing.
Stents
TCC can block the flow of urine into and out of the bladder. Complete obstruction can rapidly lead to a buildup of urea and life-threatening complications. If urine flow is obstructed, stents (small tubes) can be placed in the ureters or urethra, as needed, to open up the “channels” and restore urine flow. Our group is working closely with our urology colleagues at Purdue, to provide this opportunity for dogs that need it. Urethral stents are typically placed with fluoroscopic guidance in a non-surgical procedure. Ureteral stents can be placed surgically, and in some cases non-surgically. Another approach to bypass urethral obstruction is to place a cystotomy tube (small diameter tube that goes from the bladder through the wall of the abdomen to the outside) to allow emptying of the bladder.
CAN TCC BE PREVENTED?
Steps that can be taken to reduce the risk of TCC in dogs, especially in dogs in high-risk breeds (Scottish terriers, West Highland white terriers, Wire hair fox terriers, Shetland sheepdogs, beagles) include: (1) avoiding older generation flea control products, i.e. flea dips and sprays, (2) preventing exposure to cigarette smoke, (3) avoiding lawns treated with herbicides and pesticides, (4) maintaining a healthy body weight and avoiding obesity, and (5) feeding vegetables at least three times per week. These will reduce the risk of TCC, although some dogs will still develop the cancer if these recommendations are followed. There are other causes for TCC that have not yet been identified. The biggest risk factor for bladder cancer in humans is smoking. The role of exposure to cigarette smoke in bladder cancer risk in dogs has also been found in recent studies. It is best to limit exposure to smoke in dogs as it could increase cancer risk and cause other disease in dogs too.”
TCC SCREENING FOR EARLY DETECTION
There is a lot of interest in screening for bladder cancer and finding it earlier. Theoretically when comparing “early” cancer detected through screening before any symptoms or outward evidence of cancer emerge, to “later” cancer found once dogs have bothersome symptoms and bladder dysfunction, the “early” cancer should be more amenable to treatment. The early cancer should be associated with better antitumor immune responses, better blood flow to allow drug delivery to the tumor, less molecular changes including less drug resistance pathways, and better health of the dog to allow treatment. Plus, if the dog has no symptoms of the cancer, then if the cancer is just halted in its progression, the dog will continue to be symptom free. The study and the results are discussed in more detail at New study published on bladder cancer screening and early treatment in dogs. Briefly, 120 Scottish terriers were screened for 3 years with urinary tract ultrasound, urinalysis with urine sediment exam, and cystoscopic biopsies in dogs with positive screening tests. The major “take home” results are: (1) it is possible to find bladder cancer early while it is still small, organ-confined, and not causing any symptoms yet, (2) early cancer does respond much better to treatment than later cancer, (3) studies of the molecular makeup of the cancer reveal clues to the factors driving the progression of cancer, (4) the early and later canine tumors are similar to high grade urothelial carcinoma in humans, and (5) urine tests based on specific tumor antigens and mutations such as the BRAF mutation had a high rate of false positive results meaning these tests were not found to be useful in predicting the presence or the emergence of bladder cancer in dogs.
For more information please see New study published on bladder cancer screening and early treatment in dogs.
WHAT CAN BE LEARNED FROM DOGS WITH TCC THAT WILL HELP HUMAN CANCER PATIENTS?
In the Werling Comparative Oncology Research Center (WCORC) at Purdue University, we study specific forms of naturally-occurring cancer in pet dogs in order to learn new information to help animals and to help human cancer patients. This is possible because certain naturally occurring canine cancers greatly resemble that same form of cancer in humans. This is true with bladder cancer. Canine TCC is almost identical to human invasive TCC (invasive urothelial carcinoma) in histopathologic characteristics, molecular features studied to date, biologic behavior (sites and frequency of metastasis), and response to medical therapy. Our laboratory is studying the risk factors (environmental and genetic) for TCC, methods to detect TCC earlier, and methods to more effectively treat TCC. These studies are expected to benefit both animals and humans with cancer. In fact, our work has already led to clinical trials in humans with TCC.
CANINE BLADDER CANCER CLINIC AT PURDUE UNIVERSITY
Within the Oncology Section in the Purdue University Veterinary Hospital, a Canine Bladder Cancer Clinic has been established to care for dogs that have TCC or dogs suspected of having TCC. The clinic is staffed by Dr. Deborah Knapp, other veterinarians specializing in oncology, veterinary technicians, and assistants. Diagnostic procedures including cystoscopy, cancer staging tests, and multiple types of therapy are offered. The Canine Bladder Cancer Clinic also typically has ongoing clinical trials to help dogs with TCC while learning new information that can help other dogs and potentially humans with this cancer. Veterinarians in the Bladder Cancer Clinic work closely with primary care veterinarians in the dog’s home town to provide the most complete care.
If you do come to see us at the Bladder Cancer Clinic, please plan on a full-day visit and if cystoscopy is indicated, then please plan on a two-day visit. Our oncology client liaisons who can be reached at 765-494-1107, can assist you in making an appointment. Please note that we are often overbooked, and we ask that you please be patient with us if you have to wait. Thank you!
Recent Publications and Selected Earlier Publications Related to Bladder Cancer:
-
Knapp DW, Dhawan D, Ruple A, Cooper BR, Zhang M, Liu D, Ramos-Vara JA, Bonney PL, Fourez LM, Enstrom AW, Lahrman SA, Tullius JA. Association between cigarette smoke exposure and urinary bladder cancer in Scottish terriers in a cohort study. Vet J. 2023 Nov 23;303:106044. Epub ahead of print. PMID: 38000695.
-
Long J, Ganakammal SR, Jones SE, Kothandaraman H, Dhawan D, Ogas J, Knapp DW, Beyers M, Lanman NA. cTULIP: application of a human-based RNA-seq primary tumor classification tool for cross-species primary tumor classification in canine. Front Oncol. 2023 Jul 20;13:1216892.
- Dhawan D, Ramos-Vara JA, Utturkar SM, Ruple A, Tersey SA, Nelson JB, Cooper BR, Heng HG, Ostrander EA, Parker HG, Hahn NM, Adams LG, Fulkerson CM, Childress MO, Bonney PL, Royce C, Fourez LM, Enstrom AW, Ambrosius LA, Knapp DW. Identification of a naturally-occurring canine model for early detection and intervention research in high grade urothelial carcinoma. Front Oncol. 2022;12:1011969.
- Varvil MS, Bailey T, Dhawan D, Knapp DW, Ramos-Vara JA, Dos Santos AP. The miRNome of canine invasive urothelial carcinoma. Front Vet Sci. 2022;9:945638. Heng HG, Ramos-Vara JA, Fulkerson CM, Fourez LM, Knapp DW. Ultrasonographic detection of apex nodules in the urinary bladder of Scottish Terriers. Vet Radiol Ultrasound. 2022 Mar;63(2):234-239.
- Sommer BC, Dhawan D, Ruple A, Ramos-Vara JA, Hahn NM, Ostrander EO, Parker HG, Fulkerson CM, Childress MO, Fourez LM, Enstrom AW, Knapp DW. Basal and luminal molecular subtypes in naturally-occurring canine urothelial carcinoma are associated with tumor immune signatures and dog breed. Bladder Cancer 2021; 7:317-333.
- Rossman P, Zabka TS, Ruple AA, Tuerck D, Ramos-Vara JA, Liu L, Mohallem R, Merchant M, Franco J, Fulkerson CM, Bhide KP, Breen M, Aryal UK, Murray E, Dybdal N, Utturkar SM, Fourez LM, Enstrom AE, Dhawan D, *Knapp DW. Phase I / II trial of vemurafenib in dogs with naturally-occurring, BRAF-mutated urothelial carcinoma. Mol Cancer Ther. 2021 Nov;20(11):2177-2188.
- Woolcock AD, Cheney A, Deshuillers P, Knapp D, Moore GE. Assessment of urinary 15-F2 -isoprostanes in dogs with urothelial carcinoma of the urinary bladder and other lower urinary tract diseases. J Vet Intern Med. 2020 Nov;34(6):2454-2459.
- Knapp DW, Dhawan D, Ramos-Vara JA, Ratliff TL, Cresswell GM, Utturkar S, Sommer BC, Fulkerson CM, Hahn NM. Naturally-occurring invasive urothelial carcinoma in dogs, a unique model to drive advances in managing muscle invasive bladder cancer in humans. Frontiers in Oncology 2020; pub 21 Jan 2020, doi:10.3389/fonc.2019.01493.
- Parker HG, Dhawan D, Harris AC, Ramos-Vara JA, Davis BW, Knapp DW, Ostrander EA. RNAseq expression patterns of canine invasive urothelial carcinoma reveal two distinct tumor clusters and shared regions of dysregulation with human bladder tumors. BMC Cancer. 2020 Mar 24;20(1):251.
- Jack S, Madhivanan K, Ramadesikan S, Subramanian S, Edwards DF 2nd, Elzey BD, Dhawan D, McCluskey A, Kischuk EM, Loftis AR, Truex N, Santos M, Lu M, Rabideau A, Pentelute B, Collier J, Kaimakliotis H, Koch M, Ratliff TL, Knapp DW, Aguilar RC. A novel, safe, fast, efficient treatment for her2-positive and negative bladder cancer utilizing an EGF-anthrax toxin chimera. Int J Cancer. 2019 Oct 4. doi: 10.1002/ijc.32719. [Epub ahead of print]. PMID: 31584195.
- Chand D, Dhawan D, Sankin A, Ren X, Lin J, Schoenberg M, Knapp DW, Zang X. Immune checkpoint B7x (B7-H4/B7S1/VTCN1) is over expressed in spontaneous canine bladder cancer: The first report and its implications in a preclinical model. Bladder Cancer. 2019;5(1):63-71. doi: 10.3233/BLC-180204. PMID: 30854414.
- Dhawan D, Hahn NM, Ramos-Vara JA, Knapp DW. Naturally-occurring canine invasive urothelial carcinoma harbors luminal and basal transcriptional subtypes found in human muscle invasive bladder cancer. PLoS Genet. 2018;14(8):e1007571. PMID: 30089113.
- Szigetvari NM, Dhawan D, Ramos-Vara JA, Leamon CP, Klein PJ, Ruple AA, Heng HG, Pugh MR, Rao S, Vlahov IR, Deshuillers PL, Low PS, Fourez LM, Cournoyer AM, Knapp DW. Phase I/II clinical trial of the targeted chemotherapeutic drug, folate-tubulysin, in dogs with naturally-occurring invasive urothelial carcinoma. Oncotarget. 2018;9(97):37042-37053. doi: 10.18632/oncotarget.26455.
- D'Hue CA, Dhawan D, Peat T, Ramos-Vara J, Jarmusch A, Knapp DW, Cooks RG. Fatty acid patterns detected by ambient ionization mass spectrometry in canine invasive urothelial carcinoma from dogs of different breeds. Bladder Cancer. 2018;4(3):283-291.
- Sommer BC, Dhawan D, Ratliff TL, Knapp DW. Naturally-occurring canine invasive urothelial carcinoma: A model for emerging therapies. Bladder Cancer. 2018;4(2):149-159.
- Honkisz SI, Naughton JF, Weng HY, Fourez LM, Knapp DW. Evaluation of two-dimensional ultrasonography and computed tomography in the mapping and measuring of canine urinary bladder tumors. Vet J. 2018;232:23-26. doi: 10.1016/j.tvjl.2017.12.008. Epub 2017 Dec 12.
- Fulkerson CM, Dhawan D, Jones DR, Marquez VE, Jones PA, Wang Z, Wu Q, Klaunig JE, Fourez LM, Bonney PL, Knapp DW. Pharmacokinetics and toxicity of the novel oral demethylating agent zebularine in laboratory and tumor bearing dogs. Vet Comp Oncol. 2017 Mar;15(1):226-236.
- Fulkerson CM, Dhawan D, Ratliff TL, Hahn NM, Knapp DW. Naturally Occurring Canine Invasive Urinary Bladder Cancer: A Complementary Animal Model to Improve the Success Rate in Human Clinical Trials of New Cancer Drugs. Int J Genomics. 2017;2017:6589529.
- Charney VA, Miller MA, Heng HG, Weng HY, Knapp DW. Skeletal Metastasis of Canine Urothelial Carcinoma: Pathologic and Computed Tomographic Features. Vet Pathol. 2017 May;54(3):380-386.
- Key J, Dhawan D, Cooper CL, Knapp DW, Kim K, Kwon IC, Choi K, Park K, Decuzzi P, Leary JF. Multicomponent, peptide-targeted glycol chitosan nanoparticles containing ferrimagnetic iron oxide nanocubes for bladder cancer multimodal imaging. Int J Nanomedicine. 2016 Aug 29;11:4141-55.
- Knapp DW, Ruple-Czerniak A, Ramos-Vara JA, Naughton JF, Fulkerson CM, Honkisz SI. A Nonselective Cyclooxygenase Inhibitor Enhances the Activity of Vinblastine in a Naturally-Occurring Canine Model of Invasive Urothelial Carcinoma. Bladder Cancer. 2016 Apr 27;2(2):241-250.
- LeBlanc AK, Breen M, Choyke P, Dewhirst M, Fan TM, Gustafson DL, Helman LJ, Kastan MB, Knapp DW, Levin WJ, London C, Mason N, Mazcko C, Olson PN, Page R, Teicher BA, Thamm DH, Trent JM, Vail DM, Khanna C. Perspectives from man's best friend: National Academy of Medicine's Workshop on Comparative Oncology. Sci Transl Med. 2016 Feb 3;8(324):324ps5.
- Dhawan D, Paoloni M, Shukradas S, Choudhury DR, Craig BA, Ramos-Vara JA, Hahn N, Bonney PL, Khanna C, Knapp DW. Comparative Gene Expression Analyses Identify Luminal and Basal Subtypes of Canine Invasive Urothelial Carcinoma That Mimic Patterns in Human Invasive Bladder Cancer.
PLoS One. 2015 Sep 9;10(9):e0136688. - Knapp DW, Dhawan D, Ostrander E. "Lassie," "Toto," and fellow pet dogs: poised to lead the way for advances in cancer prevention. Am Soc Clin Oncol Educ Book. 2015:e667-72.
- Decker B, Parker HG, Dhawan D, Kwon EM, Karlins E, Davis BW, Ramos-Vara JA, Bonney PL, McNiel EA, Knapp DW, Ostrander EA. Homologous Mutation to Human BRAF V600E Is Common in Naturally Occurring Canine Bladder Cancer--Evidence for a Relevant Model System and Urine-Based Diagnostic Test. Mol Cancer Res. 2015 Jun;13(6):993-1002.
- Fulkerson CM, Knapp DW. Management of transitional cell carcinoma of the urinary bladder in dogs: a review. Vet J. 2015 Aug;205(2):217-25.
- Knapp DW, Ramos-Vara JA, Moore GE, Dhawan D, Bonney PL, Young KE. Urinary bladder cancer in dogs, a naturally occurring model for cancer biology and drug development. ILAR J. 2014;55(1):100-18.
- Schrempp DR, Childress MO, Stewart JC, Leach TN, Tan KM, Abbo AH, de Gortari AE, Bonney PL, Knapp DW. Metronomic administration of chlorambucil for treatment of dogs with urinary bladder transitional cell carcinoma. J Am Vet Med Assoc. 2013 Jun 1;242(11):1534-8.
- Knapp DW, Peer WA, Conteh A, Diggs AR, Cooper BR, Glickman NW, Bonney PL, Stewart JC, Glickman LT, Murphy AS. Detection of herbicides in the urine of pet dogs following home lawn chemical application. Sci Total Environ. 2013 Jul 1;456-457:34-41.
- Dhawan D, Ramos-Vara JA, Naughton JF, Cheng L, Low PS, Rothenbuhler R, Leamon CP, Parker N, Klein PJ, Vlahov IR, Reddy JA, Koch M, Murphy L, Fourez LM, Stewart JC, Knapp DW. Targeting folate receptors to treat invasive urinary bladder cancer. Cancer Res 2013;73:875-84.
- Knapp DW, Henry CJ, Widmer WR, Tan KM, Moore GE, Ramos-Vara JA, Lucroy MD, Greenberg CB, Greene SN, Abbo AH, Hanson PD, Alva R, Bonney PL. Randomized trial of cisplatin versus firocoxib versus cisplatin/firocoxib in dogs with transitional cell carcinoma of the urinary bladder. J Vet Intern Med 2013; 27:126–133.
- Higuchi T, Burcham GN, Childress MO, Rohleder JJ, Bonney PL, Ramos-Vara JA, Knapp DW. Characterization and treatment of transitional cell carcinoma of the abdominal wall in dogs: 24 cases (1985-2010). J Am Vet Med Assoc. 2013; 15;242:499-506.
- Dhawan D, Ramos-Vara JA, Hahn NM, Waddell J, Olbricht GR, Zheng R, Stewart JC, Knapp DW. DNMT1: An emerging target in the treatment of invasive urinary bladder cancer. Urol Oncol. 2013 Nov;31(8):1761-9.
- Zhang J, Wei S, Liu L, Nagana Gowda GA, Bonney P, Stewart J, Knapp DW, Raftery D. NMR-based metabolomics study of canine bladder cancer. Biochim Biophys Acta 2012;1822:1807-14.
- Hahn NM, Bonney PL, Dhawan D, Jones DR, Balch C, Guo A, Hartman-Frey C, Fang F, Parker HG, Kwon EM, Ostrander EA, Nephew KP, Knapp DW. Subcutaneous 5-azacitidine treatment of naturally-occurring canine urothelial carcinoma: a novel epigenetic approach to human urothelial carcinoma drug development. J Urol 2012;187:302-9.
- McMillan SK, Knapp DW, Ramos-Vara JA, Bonney PL, Adams LG. Outcome of urethral stenting for management of obstruction secondary to transitional cell carcinoma in 19 dogs (2007-2010). J Amer Vet Med Assoc 2012;241:1627-32.
- Naughton JF, Widmer WR, Constable D, Knapp DW. Accuracy of three-dimensional and two-dimensional ultrasonography in measuring tumor volume in dogs with transitional cell carcinoma of the urinary bladder. Am J Vet Research 2012;73:1919-24.
- Childress MO, Adams LG, Ramos-Vara J, Freeman LJ, He S, Knapp DW. Comparison of cystoscopy vs surgery in obtaining diagnostic biopsy specimens from dogs with transitional cell carcinoma of the urinary bladder and urethra. J Amer Vet Med Assoc 2011;239:350-6.
- McMillan SK, Boria P, Moore GE, Widmer WR, Bonney PL, Knapp DW. Antitumor effects of deracoxib treatment in 26 dogs with transitional cell carcinoma of the urinary bladder. J Amer Vet Med Assoc 2011;239:1084-9.
- Arnold EA, Childress MO, Fourez LM, Tan KM, Stewart JC, Bonney PL, Knapp DW. Phase II clinical trial of vinblastine in dogs with transitional cell carcinoma of the urinary bladder. J Vet Intern Med 2011; 25:1385-1390.
- Abbo AH, Jones DR, Masters AR, Stewart JC, Fourez L, Knapp DW. Phase I clinical trial and pharmacokinetics of intravesical Mitomycin C in dogs with localized transitional cell carcinoma of the urinary bladder. J Vet Intern Med 2010 24:1124-30.
- Dhawan D, Craig BA, Cheng L, Snyder PW, Mohammed SI, Stewart JC, Zheng R, Loman RA, Foster RS, Knapp DW. Effects of short-term celecoxib treatment in patients with invasive transitional cell carcinoma of the urinary bladder. Mol Cancer Ther 2010;9:1371-1377.
- Dhawan D, Ramos-Vara JA, Stewart JC, Zheng R, Knapp DW. Canine invasive transitional cell carcinoma cell lines: in vitro tools to complement animal model of invasive urinary bladder cancer. Urolog Oncol 2009; 27:284-292.
- Dill AL, Ifa DR, Manicke NE, Costa AB, Ramos-Vara JA, Knapp DW, Cooks RG. Lipid profiles of canine invasive transitional cell carcinoma of the urinary bladder and adjacent normal tissue by desorption electrospray ionization imaging mass spectrometry. Analytical Chemistry 2009; 81:8758-8764.
- Dhawan D, Jeffreys AB, Zheng R, Stewart JC, Knapp DW. Cyclooxygenase-2 dependent and independent antitumor effects induced by celecoxib in urinary bladder cancer cells. Mol Cancer Ther 2008, 7:897-904.
- Wilson CR, Regnier FE, Knapp DW, Raskin RE, Andrews DA, Hooser SB. Glycoproteomic profiling of serum peptides in canine lymphoma and transitional cell carcinoma. Vet Comp Oncology 2008; 6:171-181.
- Knapp DW, Adams LG, Degrand AM, Niles JD, Ramos-Vara JA, Weil AB, O'Donnell MA, Lucroy MD. Frangioni JV. Sentinel lymph node mapping of invasive urinary bladder cancer in animal models using invisible light. Eur Urol 2007; 52:1700-1708.
- Mohammed SI, Deepika D, Abraham S, Snyder PW, Waters DJ, Lu M, Wu L, Zheng R, Stewart J, Knapp DW. Cyclooxygenase inhibitors in urinary bladder cancer: in vitro and in vivo effects. Mol Cancer Ther 2006; 5:329-336.
- Abraham S, Knapp DW, Cheng L, Snyder PW, Mittal SK, Bangari DS, Kinch M, Wu L, Dhariwal J, Mohammed. Expression of EphA2 and Ephrin A-1 in carcinoma of the urinary bladder. Clin Cancer Res 2006;12:353-360.
- Boria PA, Glickman NW, Schmidt BR, Widmer WR, Mutsaers AJ, Adams LG, Snyder PW, DiBernardi L, de Gortar AE, Bonney PL, *Knapp DW. Carboplatin and piroxicam in 31 dogs with transitional cell carcinoma of the urinary bladder. Vet Comp Oncol 2005;3:73-80.
- Raghavan M, Knapp DW, Bonney PL, Dawson MH, Glickman LT. Evaluation of the effect of dietary vegetable consumption on reducing risk of transitional cell carcinoma of the urinary bladder in Scottish Terriers. J Am Vet Med Assoc 2005;22:94-100.
- Raghavan M, Knapp DW, Dawson MH, Bonney PL, Glickman LT. Topical spot-on flea and tick products and the risk of transitional cell carcinoma of the urinary bladder in Scottish Terrier dogs. J Am Vet Med Assoc 2004;225:389-94.
- Glickman LT, Raghavan M, Knapp DW, Bonney PL, Dawson MH. Herbicide exposure and the risk of transitional cell carcinoma of the urinary bladder in Scottish Terrier dogs. J Am Vet Med Assoc 2004;1290-1297.
- Mohammed SI, Craig BA, Mutsaers AJ, Glickman NW, Snyder PW, deGortari AE, Schlittler DL, Coffman KT, Bonney PL, Knapp DW. Effects of the cyclooxygenase inhibitor, piroxicam in combination with chemotherapy on tumor response, apoptosis, and angiogenesis in a canine model of human invasive urinary bladder cancer. Mol Cancer Ther 2003;2:183-188.
- Mohammed SI, Bennett PF, Craig BA, Glickman NW, Mutsaers AJ, Snyder PW, Widmer WR, DeGortari AE, Bonney PL, Knapp DW. Effects of the cyclooxygenase inhibitor, piroxicam, on tumor response, apoptosis, and angiogenesis in a canine model of human invasive urinary bladder cancer. Cancer Res 2002;62:356-358.
- Knapp DW, Glickman NW, DeNicola DB, Bonney PL, Lin TL, Glickman LT. Naturally-occurring canine transitional cell carcinoma of the urinary bladder, a relevant model of human invasive bladder cancer. Invited submission to Urol Oncol 2000;5:47-59.
- Knapp DW, Richardson RC, Chan TCK, Bottoms GD, Widmer WR, DeNicola DB, Teclaw R, and Bonney PL. Piroxicam therapy in 34 dogs with transitional cell carcinoma of the urinary bladder. J Vet Intern Med 1994;8:273-278.
- Knapp, DW, Richardson RC, Bottoms GD, Teclaw R, Chan TC. Phase I trial of piroxicam in 62 dogs bearing naturally occurring tumors. Cancer Chemother Pharmacol 1992;29:214-218.

