Vaccine Development and Adjuvants
The HogenEsch Research Group is interested in improving the efficacy of vaccines by developing new adjuvants and better vaccine adjuvant formulations.
Adjuvants (the term is derived from the Latin word adjuvare = to help) are substances added to vaccines to enhance the immune response and to induce the type of immune response that provides optimal protection following vaccination.
Aluminum-containing adjuvants are commonly used in human vaccines, but other adjuvants such as oil-in-water emulsions have recently been introduced for a few licensed vaccines.
Vaccines for use in animals contain a broad variety of adjuvants including aluminum adjuvants, oil emulsions, liposomes and polymers.
We are investigating how aluminum-containing adjuvants work and ways to improve the efficacy of these adjuvants.
A second project is focused on the development of completely new adjuvants based on plant-derived nanoparticles.
Our lab is also interested in developing vaccines to control wildlife populations such as deer and horses. Such immunocontraceptives induce immune responses that transiently reduce the fertility of animals.
Aluminum adjuvants
Aluminum adjuvants are present in many vaccines including diphtheria-tetanus-acellular pertussis (DTaP), hepatitis B, meningococcal and pneumococcal vaccines, and the human papilloma virus (HPV) vaccines.
There are two types of aluminum adjuvants, aluminum hydroxide adjuvant and aluminum phosphate adjuvant. They differ in surface charge and in structure which affects their interaction with vaccine antigens and their biological effects.
Aluminum adjuvants adsorb vaccine antigens via electrostatic and ligand exchange mechanisms. The degree and strength of adsorption can be controlled by the choice of aluminum adjuvant, the phosphate content and ionic strength of the buffer and by conjugating antigens with phosphate groups. The role of adsorption in enhancing the immune response varies and depends on the antigen and the dose of antigen.
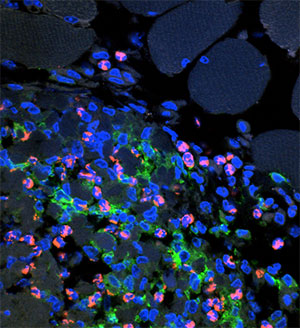
Aluminum adjuvants induce mild inflammation at the injection site following intramuscular or subcutaneous injection. The inflammation is thought to be necessary for the stimulation of the immune response by recruiting antigen-presenting cells, and is generally not harmful.
The large positively charged adsorptive surface of aluminum adjuvants allows adsorption of negatively charged immunomodulators such as nucleotides as well as antigen. This enhances the immune response while preventing unwanted systemic effects.
Related publications
- Lu F, Mosley YC, Brown D, Carmichael B, HogenEsch H (2019) Formulation of aluminum hydroxide adjuvant with TLR agonists poly(I:C) and CpG enhances the magnitude and avidity of the humoral immune response. Vaccine, 37:1945-1953.
- HogenEsch H, O'Hagan DT, Fox CB (2018) Optimizing the utilization of aluminum adjuvants in vaccines: you might just get what you want. Npj Vaccines, 3:51.
Nanoparticle adjuvants
Aluminum adjuvants have a few limitations including their susceptibility to freezing, the persistence of inflammation at the injection site for months, and sometimes negative effects on the stability of adsorbed antigens.
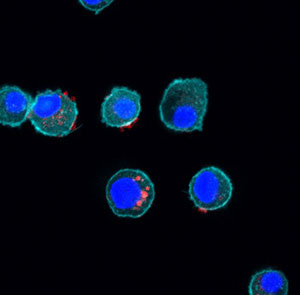
We discovered that nanoparticles derived from a variety of sweet corn, modified to give them a positive surface charge, can be used as vaccine adjuvants. The 70-80 nm particles, termed Nano-11, are readily taken up by dendritic cells and activate dendritic cells to increase the expression of CD80 and CD86 and to secrete IL-1β.
In vivo studies indicate that the nanoparticles are preferentially taken up by dendritic cells migrating to the draining lymph nodes. Nano-11 enhanced the immune response to different protein antigens in mice, pigs and horses.
Advantages over currently licensed adjuvants include stability, biodegradability and low cost.
Furthermore, Nano-11 can be used for intradermal and intranasal immunization which can induce a more effective immune response and avoids the use of needles. We recently found that Nano-11 has marked synergistic effects when it is combined with cyclic dinucleotides, which act as ligands for the intracellular receptor STING.
Related publications
- Lu F, Mencia A, Bi L, Taylor A, Yao Y, HogenEsch H (2015) Dendrimer-like alpha-D-glucan nanoparticles activate dendritic cells and are effective vaccine adjuvants. J Control Release, 204: 51-59.
- Hernandez-Franco J, Mosley YC, Franco J, Ragland D, Yao Y, HogenEsch H (2021) Effective and safe stimulation of humoral and cell-mediated immunity by intradermal immunization with a cyclic dinucleotide/nanoparticle combination adjuvant. J Immunol, 206: 700-711.
Immunocontraceptive vaccines for wildlife
Control of wildlife populations has become important as their natural habitats decrease in size and and natural predators disappear. This includes white-tailed deer in suburban areas, wild horses and burros in the western US, and feral pigs. Lethal control methods are not always effective, desirable, or safe. Alternative methods are needed to control wildlife populations.
Immunocontraception is aimed at mobilizing the immune system to reduce fertility by preventing fertilization of oocytes or by neutralizing hormones. Vaccines that induce immune responses against zona pellucida (ZP) proteins or against gonadotropin releasing hormone (GnRH) have been shown to induce transient infertility in a variety of species.
Our research is aimed at developing a next generation immunocontraceptive vaccine that is safe, easy to administer and effective.


