Research
The Pillai lab uses stem cell models to gain novel insights into mammalian development and develop applications to improve human and animal health.
Research focus in the lab can be broadly classified into the following areas:
- Pluripotent Stem Cells and Early Embryonic Development
- Trophoblast Stem Cells and Fetal-Maternal Interface
- Adult Stem Cells and 3D Cell Culture Models
Pluripotent Stem Cells and Early Embryonic Development
Pluripotency, defined as the capability to produce all cell lineages within an organism, can be captured in vitro across a variety of stem cell states under different culture conditions. These distinct stem cell states possess unique spatiotemporal traits, providing an unprecedented tool towards the study of early mammalian development. With the exception of a few rodents and primates, pluripotent stem cells (PSCs) were not derived from other mammalian species, until recently. However, those that have been derived are still not efficient in forming stable chimeras. By modulating culture conditions, we recently generated bovine PSCs with distinct molecular and functional properties. This capability opens new avenues for various agricultural and biotechnological applications, such as the generation of genetically superior livestock. We aim to define and refine culture conditions for deriving PSCs from diverse mammalian species. These PSCs will facilitate reproductive biotechnology, regenerative medicine, agricultural applications, and comparative molecular and functional analyses of early developmental programs across species. Our ongoing research explores the effects of growth factors, cytokines, extracellular substrates, and the physicochemical environment of the stem cell niche on the molecular and functional properties of cultured PSC s from multiple species. This work will enable us to create novel stable PSCs providing in vitro models to study the regulatory programs that underlie ontogenesis in vivo.
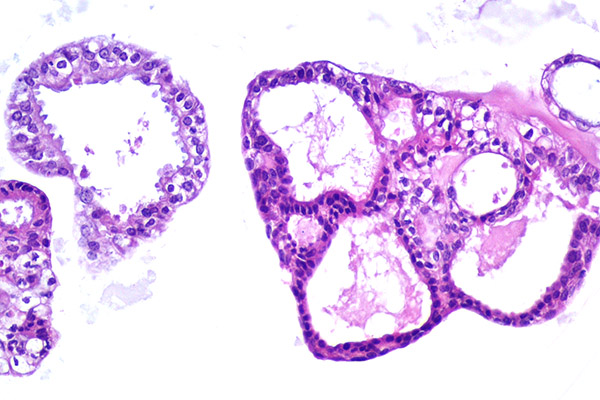
Trophoblast Stem Cells and Fetal-Maternal Interface
The placenta is essential for normal mammalian development, and successful pregnancy establishment requires appropriate reciprocal communication between the maternal uterus and the developing embryo. Any abnormalities in this cross-talk can lead to pregnancy failure. Although placentae across different clades can be considered homologous, fundamental morphological and functional cellular distinctions are apparent in both the physical enveloping of the growing fetus and the optimization of apposition between the fetal and maternal circulatory systems. Trophoblasts, the predominant epithelial cell type in the placenta, originate from the outer layer of cells in the blastocyst, called the trophectoderm. The formation of the placental interface in ruminants is unique; the bovine early embryo undergoes elongation, characterized by rapid proliferation and secretion of active factors that modify the uterine microenvironment to facilitate gestation, trophoblast differentiation, and the formation of differentiated cells at caruncular attachment sites. Despite decades of study on the bovine embryo, the mechanisms controlling the proliferation of trophoblasts and regulating their differentiation remain unknown.
Recently, we established an in vitro model system to study bovine trophoblast function and described the physiological profile of undifferentiated bovine blastocyst-derived trophoblasts/trophoblast stem cells (TSCs), highlighting the hallmarks of a self-renewing primordial state. Using this model, we identified key factors that facilitate trophoblast stem cell renewal in cattle and core signaling mechanisms that drive trophoblast proliferation and trigger differentiation. With the stem cell technologies we have developed, we aim to recapitulate placental development in vitro and understand the underlying mechanisms. We hope to use this knowledge to improve artificial reproductive technologies and develop new treatments for pregnancy complications in both humans and animals.
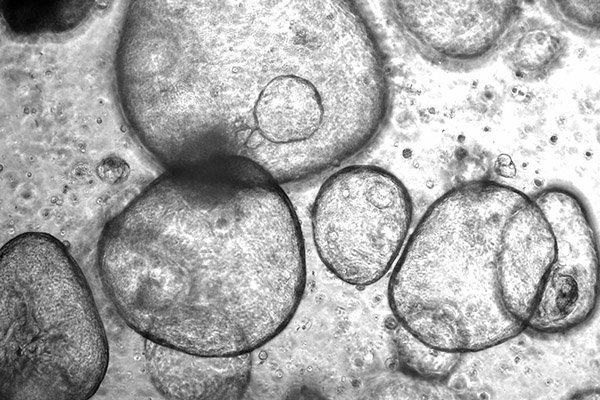
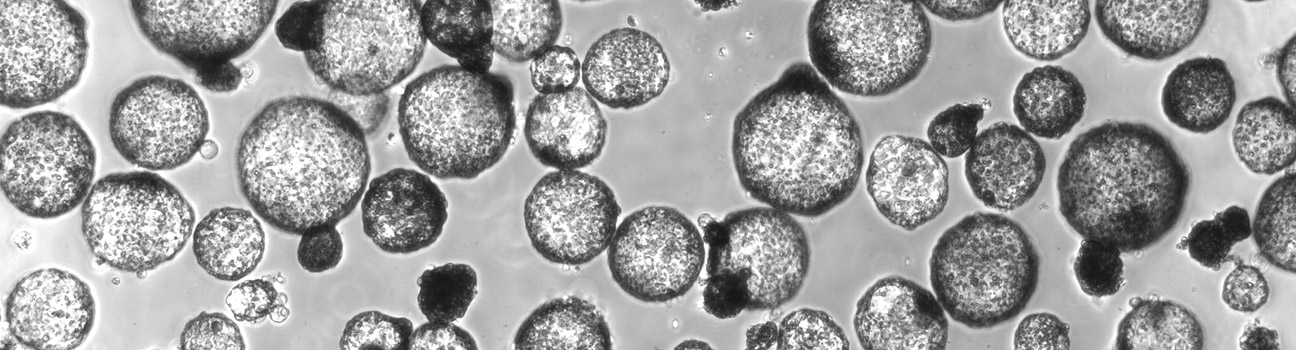
Adult Stem Cells and 3D Cell Culture Models
Three-dimensional cell culture models, including organoids derived from PSCs or adult stem cells, are capable of self-renewal, self-organization, and exhibit organ functionality. They address the limitations of existing model systems by recapitulating the in vivo cell-to-cell and cell-to-matrix interactions more effectively, providing a more complete representation of the natural environment in which the stem cells reside. Compared to traditional 2D culture models, they provide a more in vivo-like representation with higher order tissue complexity. Our group is exploring the applications of stem cell derived 3D models in cancer research, drug discovery, and the study of different types of diseases and host-pathogen interactions. Additionally, our investigations extend to optimizing the culture conditions and engineering techniques to enhance the fidelity and functionality of these 3D models in livestock and companion animals. We seek to develop novel strategies for the generation and manipulation of organoids, enabling precise control over their structure and function to unlock new avenues for understanding disease mechanisms, screening potential therapeutics, and advancing personalized veterinary medicine approaches.