Fighting superbugs using multidisciplinary strategies
Alexander Fleming’s discovery of penicillin in the 1920s paved the way to new and widely used therapeutic drugs to treat infections – antibiotics. A century later, we are now facing one of the biggest battles in global health – multi-drug resistant superbugs and limited antibiotics to control them.
Antibiotics are widely used in healthcare, veterinary medicine and animal food production industry. They are an important component of modern medicine and used to treat minor infections of the skin to life threating infectious diseases like sepsis, pneumonia, meningitis, gastroenteritis and tuberculosis. Antibiotic drugs also help fight infections for immunocompromised patients undergoing cancer therapy, organ transplants, joint replacements and treatment of chronic diseases like diabetes, asthma, and rheumatoid arthritis.
An alarming rise and spread of antibiotic or antimicrobial resistance (AMR) was observed globally in the recent decades. AMR is an adaptive ability of microorganisms, particularly disease causing bacteria, fungi and other parasites, to overcome the toxic effects of the antibiotic drugs designed to destroy them or control their growth and spread. These resistant microbes continue to grow in the presence of antibiotics making a hitherto easily treatable infection difficult to control and in certain cases impossible. According to the Centers for Disease Control and Prevention, at least 2 million Americans become infected with antimicrobial resistant bacteria each year and more than 23,000 of them die from these infections. Therefore, AMR is one of the world’s most urgent public health problems not only affecting the healthcare branch but also veterinary, and agriculture sectors.
Dr. Mohamed Seleem, Professor of Microbiology and Section head of Microbiology, Immunology and Molecular Genetics at Purdue College of Veterinary Medicine is an expert in the field of infectious diseases and his lab conducts leading research in the field of antimicrobial resistance. His research program focused on two major areas: antimicrobial resistance and drug discovery. Highly collaborative in nature, his research integrates bacteriology, mycology, molecular pathogenesis, immunology, medicinal chemistry, pharmacology, and animal models. Bringing together these different disciplines is essential for producing scientific and medical advances for difficult and critically important clinical problems such as antimicrobial drug discovery. His research tackles AMR using a multipronged approach. (i) He collaborates with experts in engineering to develop and enhance technology that can change diagnostic landscape by quick identification of drug resistant and susceptible microbes for targeted and early treatments that can improve patient outcomes; (ii) His lab works on developing new antibiotic drugs that can treat the superbugs to ensure a steady supply of medication that can control the constantly evolving superbugs and; (iii) His research team also develops alternative treatments that can substitute or supplement the use of antibiotic drugs for effective treatment of superbugs.

Professor Mohamed Seleem (Photo Courtesy of Purdue University/Rebecca Wilcox & Allison Carey)
Dr. Seleem collaborates with Dr. Ji-Xin Cheng, Professor, College of Engineering, Boston University and world-wide leader in coherent Raman scattering microscopy to develop new diagnostic methods that cut the time for detection of microbes causing Blood Stream Infections (BSI) from days to a few hours. BSIs are fatal and widespread. “In the US alone”, mentions Dr. Seleem, “BSI affects more than one million people every year, causing significant mortality ranging from 28 to 50%”. Many types of bacteria and fungi, once entering the blood stream, can multiply rapidly and are carried across the entire body causing quick manifestation of symptoms for infection. According to Dr. Seleem, “While antimicrobials remain one of the main forms of therapy, quick diagnosis of the causative pathogen is one of the main challenges”. Increased emergence and spread of antibiotic resistant microbes further increased the therapeutic challenges for BSI by increasing the risk of treatment failures, lengthening the time of diagnosis & treatment and narrowing the treatment options available.
Dr. Seleem is determined to find better and faster ways of diagnosis that can help reduce mortality associated with BSI. “In current clinical practice, patients suspected of having a bloodstream infections are initially treated with broad-spectrum antimicrobials that, in many instances, are not necessary, and can harm the patient and contribute to the emergence of antimicrobial-resistant pathogens”. Meanwhile, blood specimens are cultured for 1 to 5 days to determine the microbial identity and susceptibility to various antimicrobial drugs. Dr. Seleem says, “For every hour of delay in starting correct antimicrobial therapy, the risk of death for a given patient with sepsis increases by 6 to 10%”. Drs. Seleem and Cheng are developing a microsecond-scale stimulated Raman spectroscopic imaging platform to enable the quick identification of a single bacterium/fungus directly from the blood sample. This new imaging platform can identify molecules in their original medium, in this case blood, based on their unique vibrational fingerprints. By identifying the molecules in the biochemical pathways that distinguish a resistant strain of bacteria or fungus from the susceptible ones, Dr. Seleem’s team will not only be able to detect microbes but their response to an antimicrobial drug in minutes instead of days (Hong et al, 2018; Karanja et al, 2017). Dr. Seleem believes that this new imaging platform will change the BSI diagnosis from time-consuming, microbial cultivation dependent procedure to in situ approach, thus saving time and lives.
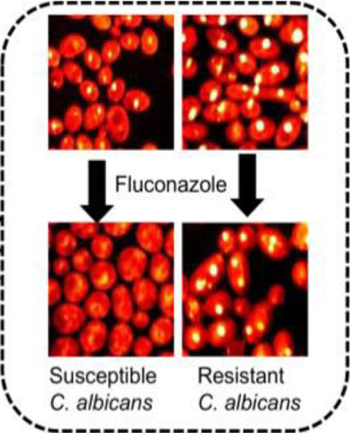 Image showing metabolic changes that can be quickly identified directly from blood samples in the presence of antibiotics using stimulated Raman scattering (SRS) imaging. The changes shown differentiate antibiotic (fluconazole) susceptible and resistant Candida albicans, most common fungal pathogen that causes bloodstream infections worldwide (Karanja et al, 2017).
Image showing metabolic changes that can be quickly identified directly from blood samples in the presence of antibiotics using stimulated Raman scattering (SRS) imaging. The changes shown differentiate antibiotic (fluconazole) susceptible and resistant Candida albicans, most common fungal pathogen that causes bloodstream infections worldwide (Karanja et al, 2017).Dr. Seleem is developing new antibiotic drugs to ensure a constant supply of medication that can control the evolving superbugs. His team focuses on both de novo drug discovery which involves developing the drug from the very beginning and also repurposing old drugs. Drug repurposing is a method of identifying new medicinal use for a preapproved or a trial drug outside its original intended use. In simple terms, it involves exploring whether a drug that is intended to treat one disease or disorder, can be used to treat another one. According to Dr. Seleem “Developing a new antibiotic is time consuming with an average time of 15 years from the time of inception. Repurposing saves time and money as these drugs have already gone through safety trails.”
Dr. Seleem collaborates with other researchers in the US and abroad in multiple antimicrobial drug discovery projects. His international collaborative effort with Dr. Abdelrahman Mayhoub (Zewail University, Egypt) led to the discovery of phenylthiazoles a new class of antibiotics and is one of the many successful de novo drug discovery research efforts from his lab (Mohammad et al, 2014; Mohammad et al, 2017). Phenylthiazoles are effective against multidrug resistant strains of Methicillin-resistant Staphylococcus aureus (MRSA), Vancomycin-resistant S. aureus (VRSA), Vancomycin-intermediate S. aureus (VISA) and Vancomycin-resistant Enterococcus (VRE). With a patent secured (US Patent 9,801,861) and research funding from National Academy of Sciences and Showalter Research Trust, Dr. Seleem is well positioned to develop this new antimicrobial drug/s that can treat superbug infections.
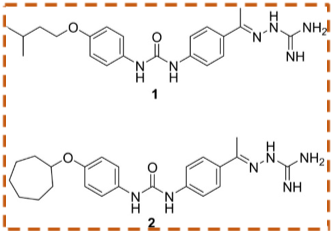 Chemical structures of two new potential antibiotics studied in Dr. Seleem’s lab that are synthetic diphenyl urea compounds and show quick control of MRSA at low concentrations while showing no toxicity to human cells (Mohammad et al, 2017).
Chemical structures of two new potential antibiotics studied in Dr. Seleem’s lab that are synthetic diphenyl urea compounds and show quick control of MRSA at low concentrations while showing no toxicity to human cells (Mohammad et al, 2017).He is currently working with research faculty in the Department of Veterinary Clinical Sciences (VCS) in the development of two repurposed drugs to control chronic eye and ear infections that are hard to treat with currently available antibiotics. These drugs are in clinical trials and are proven safe on dogs.
Prudent use of antibiotics is increasingly practiced around the world by using antibiotics as a last resort to treat infections and at the most appropriate dose for treatment. However, central to executing antibiotic prudency is the availability of viable alternatives to antibiotics that reduce or prevent disease. Dr. Seleem’s lab has developed new tool to treat MRSA infections which is reminiscent of light saber from the Hollywood movie ‘Star Wars’. The new weapon researched is blue laser light. Dr. Seleem and his team made an interesting discovery when they exposed superbugs to different colors of laser light. The bacteria that were exposed to blue light showed a color change or loss of pigments in their cell membranes. Further experiments revealed that these photo-bleached bacteria were weak and can now be killed by mild antiseptics. Furthermore unlike ultraviolet light, the blue light is perfectly safe on human skin therefore can be effectively used to treat MRSA infections. Dr. Seleem is currently working with his collaborators on developing a portable device similar to a small flash light that can treat MRSA skin infections by shining blue light on the infected area. His lab is investigating this blue light as well as other colors of laser light to control other superbugs, treat blood stream infections from multidrug resistant bacteria along with novel ways of disinfecting patients, doctors and other workers involved in health care. These alternative treatments can decrease selective pressure for the emergence and transmission of antibiotic-resistance genes in microbial pathogens.
Along with finding efficacious alternatives to reduce antibiotic usage, Dr. Seleem’s lab also works on developing novel integrative ways to improve the effectiveness of existing and new antibiotics. His research collaborations with Dr. Jean Chmielewski, a Distinguished Professor of Chemistry, and Dr. Yoon Yeo, Professor of Industrial and Physical Pharmacy at Purdue University, brings together expertise in antimicrobial resistance, peptide chemistry and drug delivery technology to target intracellular bacterial pathogens that are hard to treat.
 Blue laser light used on multi drug resistant MRSA in a contained biosafety cabinet/hood in Seleem lab to study the mechanisms of it antimicrobial activity. (photo courtesy of Purdue Research Foundation/John Underwood)
Blue laser light used on multi drug resistant MRSA in a contained biosafety cabinet/hood in Seleem lab to study the mechanisms of it antimicrobial activity. (photo courtesy of Purdue Research Foundation/John Underwood)Some bacteria like MRSA, Salmonella and Mycobacterium tuberculosis learned to live inside certain mammalian cells like macrophages, where many commonly used antibiotics cannot reach adequately to be effective. In these secure shelters, these pathogens can reside and reproduce for a lifetime in the patient causing chronic infections, while the suboptimal delivery of antibiotics into the cells can further increase the problem of antimicrobial resistance. There is a need for new drugs that can effectively target these pathogens along with carrier systems that can specifically deliver antimicrobials inside the cells inhabited by these pathogens instead of the entire body.
Drs. Seleem and Chmielewski’s collaboration worked towards development of new class of antimicrobial medicines to target these intracellular pathogens using synthetic peptides that can penetrate the cells inhabited by these pathogens and also help with breakdown of established biofilms (formed by bacteria to help tolerate antibiotics and host immune responses). They recently obtained National Institute of Health - Small Business Technology Transfer Award to expedite the preclinical development of this new class of cell-penetrating antimicrobial peptides (Nepal et al; 2018).
Dr. Seleem’s group, in collaboration with Dr. Yoon Yeo’s group, Department of Industrial and Physical Pharmacy, proposed the use of a pH-sensitive delivery system (semi-nanoparticles) to promote intracellular release of antimicrobial peptides and killing of intracellular pathogens (Brucella, Listeria, MRSA and Salmonella). The collaborative team was successful in securing NSF funding to develop new materials that may be of potential use in addressing intracellular bacterial infections (Pei et al; 2017).
In addition, he is currently collaborating with researchers in the Department of Veterinary Clinical Sciences in developing new materials for orthopedic implants to address major problems associated with plates and screws placed in joints. The goal is to find new and better materials for orthopedic implants in both people and animals that prevent biofilm formation or bacterial infections after placement.
His ground breaking research along with wide range of interdisciplinary collaborations is providing real world solutions to the global threat of antimicrobial resistance.

 By using our website you agree to our
By using our website you agree to our