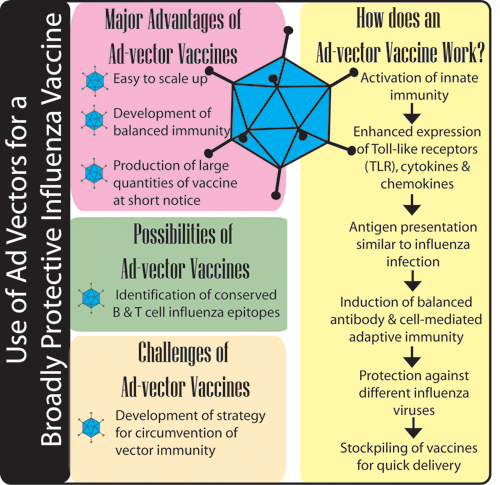
In spite of a number of advantages of human adenovirus (HAd) vectors as a delivery system for a variety of gene therapy and vaccine applications, the vector immunity is one of the major disadvantages associated with these vectors. This is due to the presence of preexisting HAd-specific immunity in the majority of the human population and the development of a vector-specific immune response following the first inoculation. HAd-specific immunity significantly lowers the efficiency of HAd vector uptake following readministration of the same vector and thus drastically reduces the levels and duration of transgene expression. Another major concern associated with HAd vectors is an acute inflammatory response and hepatotoxicity caused by activation of innate immunity. We are researching an effective strategy for circumvention of adenoviral vector immunity and toxicity for gene therapy applications.
Ahi YS, Vemula SV, Hassan AO, Costakes G, Stauffacher C and Mittal SK. (2015) Adenoviral L4 33K forms ring-like oligomers and stimulates ATPase activity of IVa2: implications and viral genome packaging. Front Microbiol. Apr 21;6:318 doi 10.3389/fmicb.2015.00318 PUBMED
Ahi YS, Vemula SV and Mittal SK. (2013) Adenoviral E2 IVa2 protein interacts with L4 33K protein and E2 DNA-binding protein. J.Gen.Virol. 94:1325-34.PUBMED
Vemula SV, Amen O, Katz JM, Donis R, Sambhara S and Mittal SK. (2013) Beta-defensin 2 enhances immunogencity and protection of an adenovirus-based H5N1 influenza vaccine at an early time. Virus Res. 178 (2):398-403. PUBMED
Vemula SV, Pandey A, Singh N, Katz JM, Donis R, Sambhara S and Mittal SK. (2013) Adenoviral vector expressing murine beta-defensin 2 enhances immunogencity of an adenoviral vector based H5N1 influenza vaccine in aged mice. Virus Res. 177:55-61. PUBMED
Sharma A, Bangari DS, Vemula SV, and Mittal SK. (2011) Persistence and the state of bovine and porcine adenoviral vector genomes in human and nonhuman cell lines. Virus Res. 161(2):181-187. PUBMED
Mittal SK, Swaim A, and Ahi YS. (2011) Adenoviral vectors: Potential and challenges as a gene delivery vehicle. In: Viral Gene Therapy. Editors: Xu K. InTech Open Access Book91-128. LINK
Harrach B, Benko M, Both GW, Brown M, Davison AJ, Echavarria M, Hess M, Jones MS, Kajon A, Lehmkuhl HD, Mautner V, Mittal SK, and Wadell G. (2011) The Double Stranded DNA Viruses: Adenoviridae. In: Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses. Editors: King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. Academic Press: Elsevier. Waltham MA. pp.125-141
Ahi YS, Bangari DS and Mittal SK. (2011). Adenoviral vector immunity: its implications and circumvention strategies. Curr. Gene Ther. 11(4):307-320. PUBMED
Sharma A, Bangari DS, Tandon M, Pandey A, HogenEsch H and Mittal SK. (2010). Evaluation of innate immunity and vector toxicity following intravenous inoculation of bovine, porcine and human adenoviral vectors in a mouse model. Virus Res. 153:134–42. PUBMED
Vemula S and Mittal SK. (2010). Production of adenovirus vectors and their use as a delivery system for influenza vaccines. Expert Opin Biol Ther. 10(10):1469-87. PUBMED
Sharma A, Tandon M, Ahi Y, Bangari DS, Vemulapalli R and Mittal S. (2010). Evaluation of Cross-Reactive Humoral and Cell-Mediated Immune Responses among Human, Bovine and Porcine Adenoviruses. Gene Ther. 2010 May;17(5):634-42. PUBMED
Sharma A, Tandon M, Bangari DS and Mittal SK. (2009) Adenovirus vector-based strategies for cancer therapy. Current Drug Therapy. 4(2): 117-138. PUBMED
Sharma A, Li X, Bangari, DS and Mittal SK. (2009). Adenovirus receptors and their implications in gene delivery. Virus Res. 143(2):184-94. PUBMED
Li X, Bangari DS, Sharma A and Mittal SK. (2009) Bovine adenovrius serotype 3 utilizes sialic acid as a cellular receptor for virus entry. Virology. 392(2):162-168. PUBMED
Sharma A, Bangari DS, Tandon M, Pandey A, HogenEsch H and Mittal SK. (2009). Comparative analysis of vector biodistribution, persistence and gene expression following intravenous delivery of bovine, porcine and human adenoviral vectors in a mouse model. Virology. 386(1):44-54. PUBMED
Bangari DS, Mittal SK. (2006). Current strategies and future directions for eluding adenoviral vector immunity. Current Gene Therapy. 6:215-226. PUBMED
Bangari DS and Mittal SK. (2006). Development of nonhuman adenoviruses as vaccine vectors. Vaccine. 24(7):849-62. PUBMED
Bangari DS, Shukla S, Mittal SK. (2005). Comparative transduction efficiencies of human and nonhuman adenoviral vectors in human, murine, bovine, and porcine cells in culture. Biochem.Biophys.Res.Commun. 327:960-966. AUTHOR MANUSCRIPT PUBMED
Bangari DS, Mittal SK. (2005). Porcine adenovirus serotype 3 internalization is independent of CAR and alpha(v)beta(3) or alpha(v)beta(5) integrin. Virology 332:157-166. LINK
Bangari DS, Mittal SK. (2004). Porcine adenoviral vectors evade preexisting humoral immunity to adenoviruses and efficiently infect both human and murine cells in culture. Virus Res. 105:127-136. AUTHOR MANUSCRIPT PUBMED